Abstracts
Abstract submission for posters is still open
We would like to offer the opportunity to both academics and industrialists to submit papers for this conference in the general field of encapsulated materials.
Our programme is complete so we can no longer accept abstracts for oral presentations. There is still the opportunity to present work in the form of a poster which will be promoted in the form of abstracts in the conference programme book and on the conference website
Submission
Abstracts should consist of a one- paragraph summary (ca 150 – 200 words) and the contact details of the authors and a contact address.
Please use the abstract template [download document]
Once complete this should be sent as a word document to the conference secretariat Constable & Smith Events by E Mail at
Deadline
The deadline for submission of oral abstracts was April 30th. Abstracts for posters should be received by October 31st. From our experience the previous two conferences were so popular that the earlier we receive these abstracts the greater the chance that they are accepted. There were 100 delegates in London and in 2016 we had over 60 delegates in Edinburgh.
The provisional programme is available to view. Follow the link on the menu to the right
Abstracts
Pharmaceutical nanotechnology involves the formation of drug loaded nanoparticles from polymers, lipids and surface active agents. Such nanoparticles have been used to formulate approved drugs, which target a particular clinical problem, such as: avoiding cardiotoxicity in the case of Doxil and avoiding hypersensitivity reactions in the case of the excipient used in Abraxane. To gain approval, provide real patient benefit and encourage prescribing, it is essential that nanomedicines are sufficiently differentiated from a clinical perspective and preclinical data should support such potential differentiation, prior to proceeding to expensive clinical testing. An increase in bioavailability, for example, is often an insufficient driver for clinical development.
Over the last two decades, we have designed a large variety of self assembling polymers and peptides and used these to develop nanomedicines, which may be administered via the intravenous oral and intranasal routes. Some of these preclinical stage nanomedicines have already demonstrated that they are well differentiated in a manner that is relevant to their clinical use. These nanomedicines show advantageous alterations in drug biodistribution and additional studies have illuminated some interesting mechanisms. These nanomedicines will be discussed in the talk.
Using solutions for fragrance oil encapsulation for developing local delivery of highly cytotoxic drugs.
Dr Olivier Cayre | University of Leeds
Encapsulation of volatile active ingredients such as fragrance oils remains a very challenging (and typically wasteful) process in many industrial applications. Standard polymer microcapsule shells are scarcely adapted for retaining such small actives because of their typical high diffusion rates, which typically means high losses of actives during processing and use.
Over the last few years, our research group has developed microcapsule shells that are impermeable to small molecules over relevant industrial timescales. These microcapsule shells consist of a thin metal shell deposited via electroless deposition methods directly onto the droplets to be encapsulated. The process is fast and allows for 100% of the actives to be retained within the microcapsules until release is activated.
In this presentation, I will introduce our methodology for producing such ‘impermable’ microcapsules and our strategies for triggering the release of the encapsulated small molecules used as model actives. I will also describe recent efforts in adapting the technology for the delivery of cytotoxic drugs.
Membrane emulsification: an innovative continuous process to create highly uniform capsules
Marijana M Dragosavac | Loughborough University, Chemical Engineering Department
In the membrane emulsification (ME) process, monodisperse droplets are generated by dispersing one liquid (dispersed phase) into a second, immiscible liquid (continuous phase) through the pores of a membrane. The droplet size and droplet size distribution are mainly determined by the membrane properties (pore size, pore size distribution, membrane wettability). The optimization of the operating conditions (dispersed phase flux and shear stress) as well as of composition of the phases (density of phases and viscosity, emulsifier type and concentration) allows the production of uniform, reproducible and size-controlled droplets. Some important challenges are required for the advancement of technologies in engineering particles with improved functional and structured properties: i) Maintaining the performance of a good emulsification step when the process is transferred from the laboratory scale to a larger throughput scale, and ii) Achieving a precise control of particle size and size distribution in the emulsification step while maintaining the incorporation of biomolecules without affecting their activity; the distribution in the body; the interaction with living cells as well as the drug release kinetics of the solid particles are greatly influenced by their size and uniformity. The ME process is attractive for the production of particles with homogeneous size distribution at large scale by increasing membrane area (Fig.1). Bench, large scale and continuous manufacturing are possible using ME.
Figure 1. Some of the highly uniform capsules produced up to date by ME (Micropore Technologies Ltd. UK (provided membranes and ME systems)
Methylselenocysteine loaded chitosan:zein nanoparticles: Formulation, characterisation, and extensive in-vitro evaluations
VOZZA Giuliana1, 2, BYRNE Hugh J. 2, RYAN Sinead M.3 and FRIAS Jesus M1.
1.School of Food Science and Environmental Health, Dublin Institute of Technology
- FOCAS Research Institute, Dublin Institute of Technology
- School of Veterinary Medicine, University College Dublin (UCD).
Selenium is an essential micronutrient and exists in different forms, both inorganic and organic. Methylselenocysteine (MSC), an organic form, has garnered attention as a selenium supplement due to its potential anti-cancer properties. There is substantial interest in developing an oral formulation of MSC, however, due to its increased susceptibility to oxidation, this has not proved possible to date. In this study, MSC was formulated into nanoparticles using the mucoadhesive polymer chitosan (Cs), via ionotropic gelation. Subsequently, the loaded MSC:Cs nanoparticles were coated with zein (maize), to obtain a higher encapsulation efficiency. The addition of zein at 0.75:1 (zein: Cs) mass ratio, resulted in NPs with an average size of 252±56.2 nm, a Polydispersity Index of 0.297±0.049, a zeta potential of 34.1±4.4 mV and an encapsulation efficiency of (80.7±4.4%). In vitro MTS cytotoxicity studies showed no decrease in cellular viability in both Caco-2 (intestine) and HepG2 (liver) cell lines after 4 and 72 hr exposures, respectively. Accelerated thermal stability of the loaded NPs indicated good stability under normal storage conditions. Lastly, after 4 hr exposure to a simulated gastrointestinal tract environment, the sustained release profile of the formulation showed that ≤ 55% of the drug had released.
Nanoencapsulation by Interfacial Polymerisation
Lionel Petton, Fabienne Goethals, Luc Decoster, Amandine Ligot, Johan Loccufier | Agfa-Gevaert NV, Septestraat 27, 2640 Mortsel, Belgium
The encapsulation technology has allowed the emergence of new and/or improved applications, where sensitive active products are protected from undesired degradation or where reactive chemicals are incorporated into a formulation and only triggered when required.
Recently, Agfa has developed new nanocapsules, which release their cargo when exposed to higher temperatures. The incorporation in inkjet inks or coatings of such capsules can provide good adhesion onto various substrates for example. In other applications, capsules are used to develop an image when exposed to laser marking.
A versatile method to produce nanocapsules is through emulsion solvent evaporation and interfacial polymerisation, whereby the shell of the capsule is in-situ polymerised at the interface between oil-droplets containing the active product to be encapsulated and an aqueous medium. Thus, the production of the nanocapsules first involves the emulsification by high-shear mixing of an oil-phase containing ethyl acetate, an isocyanate compound, as reactive shell material, and the active product (e.g. a dye or blocked isocyanate) into a water medium containing a suitable dispersing agent. The solvent is then evaporated and a catalyst added to initiate the polymerisation of the capsule shell. Typically, capsules with a particle size distribution below 1 μm and a narrow distribution are obtained. Moreover, the nanocapsules exhibit a high colloidal stability.
Metal-organic framework capsules
Darren Bradshaw | School of Chemistry, University of Southampton
Microporous metal-organic frameworks (MOFs) assembled and sustained by coordination bonds have many desirable properties for encapsulation technologies, including high surface areas and ordered porosity, tuneable functionality and pore size and in some cases stimuli-responsive behaviours. MOFs are typically prepared as microcrystalline powders and the current challenge is to develop synthetic routes to configure MOFs into capsular structures in order to exploit the unique opportunities they present. In this presentation I will discuss hard and soft templating strategies for micron-sized MOF capsules1,2 (Figure 1) and demonstrate how these can be utilized for pH-triggered on-demand release of cargo molecules. Further, the encapsulation of functional biomolecules permits the MOF capsules to be employed as recyclable size-selective biocatalysts.3
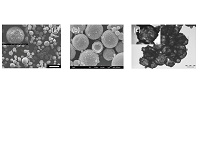
Figure 1. Electron microscopy images of MOF microcapsules prepared by soft templating around emulsion droplets (a,b) and hard templating around an oxide microsphere and subsequent template removal (c).
References:
- El-Hankari et al, J. Mater Chem A 2016, 4, 13509
- Huo et al, Adv Mater 2013, 25, 2717
- J. Huo et al, Chem Sci 2015, 6, 1938
Mesoporous CaCO3 crystals - a powerful tool for bio-friendly encapsulation
Dmitry Volodkin | Nottingham Trent University
The mesoporous vaterite CaCO3 micro- and nano-crystals are nowadays widely used as sacrificial templates for fabrication of various types of carriers, e.g. matrix and hollow capsules, porous and compact beads. Such carriers can be made from a variety of biomolecules (proteins, nucleic acids, enzymes, etc) or can host the biomolecules as a capsule load to be released in a controlled manner. The intensive use of CaCO3-based templating is driven by i) highly developed mesoporous crystal structure, ii) low preparation costs and easy lab synthesis, iii) mild crystal elimination conditions (pH<7 or chelating agents such as EDTA, citric acid). Size of the crystals can be varied starting from a few hundreds of nanometers and up to tens of microns that defines a variety of sizes of particulate biomolecules. Compactly packed capsules can form cm-sized 3D scaffolds assembled using microfluidics. Shape of the crystals can also be adjusted (spherical, ellipsoid-like, etc). Crystal porosity can be controlled without any additives by variation of the crystal preparation temperature that is driven by Ostwald ripening. This talk aims to demonstrate employing the crystals for templating polymer-based 2D and 3D structures. Inherent crystal biocompatibility, control over size and shape, and possibility to scale the porosity make the CaCO3 crystals extremely attractive tools for template assisted design of tailor-made biopolymer-based architectures targeted at drug delivery and other bio-applications.
Energy Nanocapsules for Thermo-Regulating Paints and Textiles
Dmitry Shchukin, Paula Felix de Castro | Stephenson Institute for Renewable Energy, University of Liverpool, L69 7ZF, Liverpool, UK
Innovation of new materials and technologies for storage and controlled release of the energy avoiding its unexpectable losses is critical for future green economy involving implementation of the renewable energy sources. This is particular important for storage of the thermal energy in domestic applications. Around 27 % of the energy consumption in EU in 2013 belongs to household usage, which is 295.9 million tons of oil equivalents. 67 % of the household energy is deployed for space heating, which is 18.1 % of the total EU energy consumption or 198.3 million tons of oil equivalents. In our work, we employ encapsulation of the phase change materials (PCMs, both inorganic crystallohydrates and organic paraffins) into the capsules with polymer or carbon shells as a tool for delivery of heat uptake/release properties. New encapsulation approaches have been developed and energy capsules containing PCMs have been designed (see Shchukin et al. ACS Nano, (2016), 4695; ACS Nano, (2017), DOI: 10.1021/acsnano.6b07126; Chem. – A European J., (2016), 4389; J. Mater. Chem. A, (2016), 16906). Studied energy capsules have high cycling stability during upload and release of heat (hundreds of cycles) and stable at temperatures up to 200 °C. The fundamental knowledge on encapsulation of the PCMs creates a new platform for their application as thermal energy storage additive to the commercially available paints and textiles. Such thermo-regulating paint formulations (textiles) can be used for retrofitting of the already existing buildings improving their thermal efficiency without deterioration of the architectural style, historical heritage and other functionalities. Nanocapsules and corresponding thermo-regulating paints and textiles have been characterised by DSC, TGA, SEM, Confocal Raman microscopy, FTIR and sun light thermal simulator.
Encapsulating living microorganisms via ice-templating
Sarah CHRISTOPH, Thibaud CORADIN, Francisco M. FERNANDES
Sorbonne Universités, UPMC Univ Paris 06, CNRS, Collège de France, Laboratoire de Chimie de la Matière Condensée de Paris (LCMCP), 4 Place Jussieu, Paris, France
From microencapsulation[1] to inkjet printing technology[2], a vast array of approaches have been proposed to address the need to formulate the new generation of cellularized materials. Here we will present recent results in the application of freeze casting, a materials processing technique we have adapted to encapsulate metabolically active cell-containing materials with controlled morphology[3,4]. Our approach stands at the interface between standard cryopreservation techniques and commonly used ice templating techniques, providing solid state cellularized materials with finely controlled macroporous morphology. Using a prokaryote (Pseudomonas aeruginosa) and an eukaryote (Saccharomyces cerevisiae) as model microorganisms, we show that it is possible to encapsulate living cells within polysaccharide foams using ice templating, in absence of commonly used cryoprotectants. Moreover we focus on the interplay between the processing conditions, material morphology and cellular viability to discuss the application potential of ice templating as an encapsulation strategy for the elaboration of cellularized materials. Finally, we will discuss the emerging applications of these “living” materials as biodegradation devices that benefit from the biocatalytical activity of the encapsulated species as well as the enhanced capillary transport due to the aligned macroporosity.
Metal–Organic Frameworks (MOFs): a novel host platform for enzymatic immobilisation
Clemence Sicard,a Effrosyni Gkaniatsou,a Christian Serreb and Nathalie Steunoua
a Institut Lavoisier de Versailles, UVSQ, CNRS, Université Paris-Saclay, Versailles, France ; b Institut des Matériaux Poreux de Paris, FRE 2000 CNRS-ENS-ESPCI, Paris, France
Metal-Organic Frameworks (MOFs) are porous crystalline hybrid solids, built up from an almost infinite combination of inorganic subunits and organic polytopic linkers (carboxylates, azolates). Their versatile chemical and structural features make them promising materials for numerous applications, such gas storage and separation, catalysis, biomedicine…1 Recently, this class of hybrid materials has emerged as a new type of immobilization matrix for biomacromolecules such as enzymes. Indeed, due to their tuneable physico-chemical properties (polar/apolar balance, pore size and shape, pore volume), they are perfectly suited to create a stabilizing microenvironment for enzymes through specific hydrophilic/hydrophobic interactions and/or confinement effect.2 We will present the use of nanoparticles of MIL-101 (MIL=Material Institute Lavoisier)3 as an immobilisation matrix for the enzyme microperoxidase-8 (MP8). Taking advantage of the two different mesoporous cages present in MIL-101, MP8 was encapsulated in the large cavity while keeping the smaller cavities for the pre-concentration of reactants and the diffusion of analytes (Figure). The comparison of catalytic activity, stability and reusability of the immobilised MP8 with the free enzyme will also be presented.
Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder
Daulet Kaldybekov,a Prasopchai Tonglairoum,a,b Vitaliy Khutoryanskiya
a School of Pharmacy, University of Reading, Reading, RG6 6AD, Berkshire, UK
b Faculty of Pharmacy, Silpakorn University, Nakhon Pathom, 73000, Thailand
Liposomes are microscopic vesicles composed of phospholipid bilayers with the size range from 30 nm up to several microns. Due to their specific structure, liposomes can encapsulate hydrophilic drugs in the aqueous interior and lipophilic drugs in the membrane.
Maleimide-functionalised polymers with enhanced mucoadhesive properties were previously reported as novel drug delivery vehicles by Tonglairoum et al. [1]. In this study, liposomal formulations of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (PEG2000-DSPE) and its maleimide derivatives (PEG2000-DSPE maleimide) among with conventional liposomes (CL) were prepared by conventional “film method” followed by hydration and bath sonication. These liposomes were characterised using dynamic light scattering, zeta-potential measurements and transmission electron microscopy. Sodium fluorescein (NaFI) was used as a model hydrophilic compound for encapsulation into these liposomes. It was demonstrated that PEG2000-DSPE maleimide liposomes have greater retention on porcine urinary bladder mucosa in vitro compared to CL and withstand wash out effect caused by periodic irrigation with artificial urine solution. Furthermore, in vitro release kinetics of NaFl was studied.
Microfluidic templating of functional polymer particles and capsules
João T. Cabral
Department of Chemical Engineering, Imperial College London, SW7 2AZ
Directed solidification of droplets of polymer solutions and mixtures provides a powerful and versatile platform for the generation of functional particles and capsules, whose applications range from pharmaceuticals and catalysis to paints, food and personal care. Control over capsule dimensions, shape, internal structure and overall architecture can be accessed by a predictive combination of thermodynamic and non-equilibrium processes. We employed droplet extraction or ‘evaporation’ to engineer polymer capsules from liquid droplets via a succession of steps comprising: (i) solvent exchange, (ii) demixing and coarsening, (iii) phase inversion, (iv) skin formation and eventual kinetic arrest. Analogous approaches are extensively used in membrane fabrication and opposing jet precipitation, but prior droplet templating opens unique composition pathways and gradients for particle design. Due to its versatility, we employ microfluidic approaches for generation of simple and complex emulsions, and have explored the extraction of near polymer and polymer composite mixtures. Extraction takes place by immersion into a selective solvent which is carefully chosen to be a non-solvent for the solute, a solvent for the carrier phase, and partially miscible with the droplet solvent. Typical microfluidic droplets range from 1-1000 microns in size and the resulting capsules dimensions and formation timescales scale primarily with solution concentration and droplet size, while polymer mass and architecture play a comparably smaller role. Conceptually, the process can be described as a competition between droplet shrinkage and demixing: if droplet shrinkage takes place rapidly, at high Péclet number, a polymer-rich skin is formed early in the process resulting in larger particles with great cargo capacity. Conversely, slow extraction, induced by a poor selective solvent for instance, can yield denser and uniform capsules. We introduce a quantitative descriptive model and examine the role of ternary solution thermodynamics, polymer architecture, solvent/non-solvent affinity, and impact of payload for a range of organic and inorganic cargo, as well as the impact of microstructure on dissolution and release.

[1] T. Watanabe, C. G. Lopez, J. F. Douglas, T. Ono, and J. T. Cabral, Langmuir 30 (9), 2470 (2014).
[2] C. E. Udoh, V. Garbin, and J. T. Cabral, Langmuir 32 (32), 8131 (2016)
[3] C. E. Udoh, J. T. Cabral, and V. Garbin, in press (2017)

