Click here for guidelines for preparing your poster or check the bottom of this page.
Optimising the Performance of Thermogelling Materials Using a Design-of-Experiments approach
Mohamad Abou-Shamat, Jesus Calvo-Castro, Jacqueline Stair, Michael Cook
Generic computer-aided methods for designing high-value chemicals, mixtures and formulated products
Suela Jonuzaj, Claire S. Adjiman
Pragmatic and Model-free Digital Formulation
David Nicolaides
Alginate-layered Silicate Composite Beads for the Sustained Release of Theophylline
Uttom Nandi, Dr. Vivek Trivedi
A Quality by Design Approach for the Formulation and Optimisation of Controlled-release Tablets composed of Xanthan Gum and Chitosan
Suha Dadou, Iyad Rashid, Milan Antonijevic, Babur Chowdhry, Adnan Badwan
Use of Online Near Infrared Spectroscopy as a Process Sensor for the Viscosity of Shampoo
Kiran Haroon, Philip Martin, Thomas Rodgers, Ćesar Mendoza, Michael Baker
Preparation of high quality pharmaceutical salt by extrusion processing
Md Sadeque Mithu, Steven Ross, Dennis Douroumis
3D bioprinting of chitosan based hydrogels for skin regeneration
Forough Hafezi, Susan Shorter, Joshua Boateng, Dennis Douroumis
Maximising Foamability in Ethanol-Water Mixtures
Niall Ward-O’Brien, Anthony Ryan
The Oral Delivery of -Globulin using Solid Core Drug Delivery Systems (SCDDS) Prepared via Supercritical Fluid Technology (SCFT)
Adejumoke Lara Ajiboye, Vivek Trivedi
Optimising the Performance of Thermogelling Materials Using a Design-of-Experiments approach
Mohamad Abou-Shamat, Jesus Calvo-Castro, Jacqueline Stair, Michael Cook
University of Hertfordshire
“Thermogelling” polymers exhibit a solution to gel transformation in aqueous solution above a certain temperature. Materials undergoing this transition in a physiological temperature range have been drawing attention for their use in biomedical applications as in-situ gelling systems, where the polymer solution may pass through an applicator before forming a gel upon contact with the body. Poloxamer P407 (or pluronic F127) has been widely investigated due to its “thermogelling” properties and its previously used in FDA approved medicines. However, there are acknowledged drawbacks for thermogelling poloxamer solutions that limit its performance and furthermore warrant this study: low gel strength, instability, and rapid dissolution. To improve gel strength, researchers have studied blends of poloxamer with other hydrophilic polymers, however these studies are typically limited to binary blends, and only a handful of additives have been explored. This approach is particularly attractive in pharmaceuticals as chemical modification would increase regulatory risk.
In order to optimise complex polymer blends containing poloxamer, a Taguchi Orthogonal Array (OA) design-of-experiments approach was taken. OA-L18 was performed at 8 factors (polymers) and different levels (concentration) using Design Expert® software. We examined the addition of Poly(vinyl alcohol), Polyacrylic Acid, Polyethylene glycol (each at two different molecular weights) and poloxamer 188 at three levels (0, 0.1 and 1 % w/w) to P407 at 15 and 20 % w/w. The properties of the blends were evaluated by the gelling temperature and the storage modulus (G`), which have been studied by rheometry.
Improving the gel strength of poloxamer formulations is highly important for their success in drug delivery applications. In this research project, DOE has been used to further understand the effect of commonly used polymers in complex polymer mixtures bearing P407. The effects of polymer additives have shown some significance on gelling temperature and gel strength. it is anticipated that this work will be of significant interest to those engaged in the development of safe thermogelling materials with improved performance.
Generic computer-aided methods for designing high-value chemicals, mixtures and formulated products
Suela Jonuzaj, Claire S. Adjiman
Centre for Process Systems Engineering, Department of Chemical Engineering, Imperial College London, London SW7 2AZ, U.K.
The design of chemicals and mixtures is an essential activity across a wide range of chemical engineering applications, from separation processes to product design. In the process industry, for example, suitable solvent mixtures are used in separation and purification processes, to achieve better energy efficiency, improved process economics and lower environmental impact. Mixtures are also critically important in the high-value manufacturing of chemical products, such as personal care products, pharmaceuticals and agrochemicals, where a large number of compounds are combined in formulations to obtain products with different functionalities/qualities. In current industrial practice, methods for identifying suitable chemicals and the accompanying processing technology are mainly experimental or based on database searches, which often result in a small search space due to increased cost and the finite time and resources available. Therefore, systematic methods for mixture and product design to transform trial-and-error practice into an efficient search through the vast space of possible mixtures are required to enhance sustainability and innovation.
In this work, we present a general systematic methodology for mixture and product design within the Computer-aided mixture/blend design framework. In the proposed approach, the main design decisions of the mixture/product problem (how many compounds; which specific chemicals should be used; and in what proportions) are determined simultaneously and optimally, rather than through rules of thumb. Furthermore, the molecules are designed from an extensive set of atom groups (building blocks), avoiding the use of restricted datasets. Advanced optimisation techniques are used to formulate and solve the general mixture problem. The proposed methodology is applied to the design of solvent mixtures for the crystallization of a drug and for separating chemicals via liquid-liquid extraction. Next, the design of formulated products is investigated, where the best environmentally friendly ingredients of an adhesive formulation and their proportions are identified at the same time.
Pragmatic and Model-free Digital Formulation
David Nicolaides
Dassault Systèmes BIOVIA
Imagine the goal of an “Amazon for Formulators” (if you are interested in that formulation then the following formulations may also be of interest to you). Underpinning this is the calculation of similarity, which for formulations is shockingly immature compared with the same calculation for other chemical concepts such as molecules and reactions.
We illustrate how progress can be made against this goal via quite simple digital representations of formulations. These play a similar role to a “fingerprint” for a molecule or a reaction.
Such fingerprints not only allow for progress to be made towards a robust calculation of formulation similarity, but also towards realization of other concepts which, mutatis mutandis, can be taken over from cheminformatics. These include “maximum common sub-formulation” and “matched formulation pairs”.
Attention is drawn to the fact that these are all model-free approaches, with certain specific beneifts. A short comparison with model risk is also made.
Alginate-layered Silicate Composite Beads for the Sustained Release of Theophylline
Uttom Nandi, Dr. Vivek Trivedi
University of Greenwich
The desire to maximise therapeutic activity and stability of a drug molecule with minimal side effect has become an important aspect of pharmaceutical drug delivery systems. Novel drug delivery systems based on inorganic clay materials, also known as “clay based drug delivery systems’’ are currently gaining interest within pharmaceutical community.(Suresh et al.) Layered hydroxide silica are negatively charged particles which are especially adept at retaining positively charged molecules using cationic exchange. The layered silicates are known to accommodate polar compounds between the layers to form intercalated composites. Present study involves a synthetic nanoclay (Laponite RDS®) and a model drug theophylline. The clay-drug intercalated composites showed a drug loading of 58 mg/g. The interaction between these two entities also showed an increment of the basal spacing of layered silicates from 12.9 to 15.5 Å. Drug clay hybrid particles were coated by alginate polymer using egg box model to control the drug release. FTIR analysis of composite particles showed shift in peak intensities and positions when compared to pure alginate and nanoclay, indicating possible molecular interaction. The in vitro drug release experiment was carried out and drug-clay intercalates resulted in burst release of 35% in the gastric environment, in comparison only 5% theophylline release was observed from the clay-drug-alginate composite beads. The drug release from the beads was also slower in simulated intestinal fluid which demonstrates that alginate beads were able to control the drug release from the hybrid particles evolving possibilities for targeted drug delivery to upper small intestine.
Reference:
Suresh R., Borkar SN, Sawant VS. et al Nanoclay drug delivery system. Int J pharm Sci Nanotechnology. 2010; 3(2):901-905.
A Quality by Design Approach for the Formulation and Optimisation of Controlled-release Tablets composed of Xanthan Gum and Chitosan
Suha Dadou1, Iyad Rashid2, Milan Antonijevic1, Babur Chowdhry1, Adnan Badwan2
1 Department of Pharmaceutical, Chemical & Environmental Science, Faculty of Engineering & Science, University of Greenwich, Medway Campus, Chatham Maritime, Kent ME4 4TB, UK.
2 Research and Innovation Centre, The Jordanian Pharmaceutical Manufacturing Company (JPM), P.O. Box 94, Naor 11710, Jordan.
Recently, there has been a growing interest in the use of chitosan/xanthan gum matrices to retard the release of pharmaceutical active ingredients. They are natural occurring polymers offering the advantage of abundance, biodegradability and cost-effectiveness. Much was reported in the literature on chitosan-xanthan gum hydrogels as controlled-release drug carrier. However, less has been reported on the capability of their solid mixtures to control the release of active ingredients. In addition, most of the current studies were based on the trial-and-error approach to optimise the final formulation.
In this study, a systematic quality by design approach was adopted to formulate, design and optimise a solid carrier based on chitosan and xanthan gum to control the release of the drug metoprolol succinate. A bench top compaction simulator (GTP-1, Gamlen® Tableting Ltd) was used to study the compressibility and compactibility of xanthan gum and chitosan and to assess the manufacturability of their powder form. A design of experiment approach (Design Expert®) was then employed to examine the effect of the drug-to-polymer ratio, chitosan-to-xanthan gum ratio and compression force (independent variables) on the release of Metoprolol succinate (dependent variable) from the formulated tablets so that the final formulation can be optimised accordingly. This work demonstrates the capability of formulating a controlled release drug delivery system based on chitosan and xanthan gum using an accessible and simple methodology.
Use of Online Near Infrared Spectroscopy as a Process Sensor for the Viscosity of Shampoo
Kiran Haroon1, Philip Martin1, Thomas Rodgers1, Ćesar Mendoza2, Michael Baker2
1 University of Manchester, School of Chemical Engineering & Analytical Science
2 Unilever R&D, Port Sunlight
Characterising the rheological properties of materials is of great importance in many industries in terms of product quality and process economics. In the personal care industry, viscosity is a critical quality attribute which needs to be accurately and precisely controlled. There are a few inline and online technologies available for process viscosity measurements, however they have been found to have problems related to accuracy, robustness and cleaning (Barnes, 2000).
The feasibility of using near-infrared spectroscopy to provide proxy measurements for the viscosity of typical shampoo formulations is presented. Basic shampoo formulations of varying salt content were analysed using a near infrared fibre optic probe and modelled against viscosity measurements obtained on a bench-top rheometer. Differences in the infrared spectra were found in the lower absorbance regions and using partial least squares (PLS) a promising regression model was produced. Standard normal variate (SNV) was found to be the best pre-processing method, with the most variation in the spectra found in the second overtones of the CH and OH bonds. The resulting model had a RMSECV of 1.48 Pa s and a RMSEP of 2.32 Pa s.
For a preliminary exploration into the potential of NIR to detect differences in viscosity, these results look promising. With a more in-depth investigation into the limitations of the system, expanding the calibration set and applying the same principles to a pilot scale set up, the use of NIR spectroscopy could well be used to offer an efficient means of process control for shampoo manufacturing.
Barnes, H. A. (2000). A Handbook of Elementary Rheology. a Handbook of Elementary Rheology (Vol. 331). https://doi.org/10.1126/science.1201543
Preparation of high quality pharmaceutical salt by extrusion processing.
Md Sadeque Mithu, Steven Ross, Dennis Douroumis
Faculty of Engineering and Sciences, University of Greenwich, Chatham Maritime, Chatham, Kent ME4 4TB, United Kingdom
Twin screw extrusion (TSE) is being used in pharmaceutical product formation after being successfully used in the plastic and rubber industries1. Poorly water soluble APIs, ciprofloxacin (CIP) was selected with the salt former, maleic acid for the formation of salts via TSE. Molar stoichiometric ratio 1:1 was used for CIP: maleic acid. In order to assess the optimum parameters for salt formation thermal profiles, screw speed and feed rate were investigated. The extruded solid powder was analysed by Differential scanning calorimetry (DSC), where it showed a distinct endothermic peak regards to both API and salt former (Fig.1), which indicates the formation of a new product. Further analysis was performed by powder x-ray diffraction (PXRD) to confirm the formation of salts by matching with the data previously reported 2 in Cambridge structural database (CSD). The dissolution profiles of the solid (powder) forms of the drugs were performed in suitable pH buffer solutions. CIP maleic acid salt prepared by extrusion process displayed improved release profiles compared to the bulk APIs (Fig.1). Liquid assisted grinding was also used to form the CIP maleic acid salt to compare with the extrusion process.
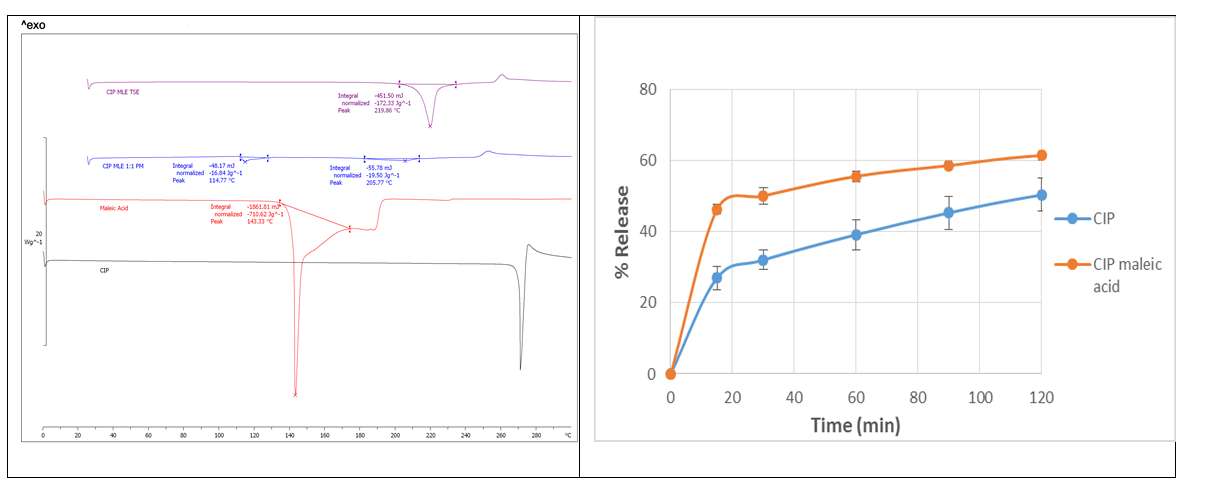
Fig.1. Thermal analysis and dissolution profiles of bulk and prepared salt.
Reference.
1. Ross, S. A., Lamprou, D. A., & Douroumis, D. (2016). Engineering and manufacturing of pharmaceutical co-crystals: a review of solvent-free manufacturing technologies. Chemical Communications, 52(57), 8772–8786.Serajuddin, A. T. M. (2007). Salt formation to improve drug solubility. Advanced Drug Delivery Reviews, 59(7), 603–616
2. Surov, A. O., Manin, A. N., Voronin, A. P., Drozd, K. V., Simagina, A. A., Churakov, A. V., & Perlovich, G. L. (2015). Pharmaceutical salts of ciprofloxacin with dicarboxylic acids. European Journal of Pharmaceutical Sciences, 77, 112–121.
3D bioprinting of chitosan based hydrogels for skin regeneration
Forough Hafezi, Susan Shorter, Joshua Boateng, Dennis Douroumis
Department of Pharmaceutical, Chemical and Environmental Sciences, Faculty of Engineering and Science, University of Greenwich, Medway, Central Avenue, Chatham Maritime, Kent, UK, ME4 4TB
Wound treatment involves both surgical and non-surgical approaches, with dressings being the most commonly employed to assist tissue repair and faster wound healing. Although current dressings on the market have been largely successful in acute wounds, it is more challenging to heal chronic wounds. Chitosan (CH) is a biocompatible and biodegradable polymer applied in pharmaceutical formulations and tissue engineering. In this research, cross-linked CH-based hydrogel films were prepared using a 3D biofabrication approach for wound healing applications. Genipin (GE) was used as a cross-linker, which unlike some synthetic chemicals is not toxic, and polyethylene glycol (PEG) was used as plasticiser. Alginate was used to support the mechanical properties of the bioprinted CH 3D structure. The CH- based hydrogels were successfully bioprinted. In vitro cell culture studies using human skin cells (human foreskin fibroblasts-HFF and keratinocyte cells-KC) showed the biocompatibility of the 3D printed constructs with human skin cells. Live/ dead assay with confocal microscopy showed that cell viability were maintained for 7 days of culturing and cells proliferated within this time frame. The proposed technique enables the possibility of bioprinting human skin cells with high survival rates and proliferation after bioprinting. This is a promising bioprinting technique that can be applied for treatment of wounds and can also find a wider application in the biofabrication of other types of tissue engineering constructs with more complex and multicellular structures.
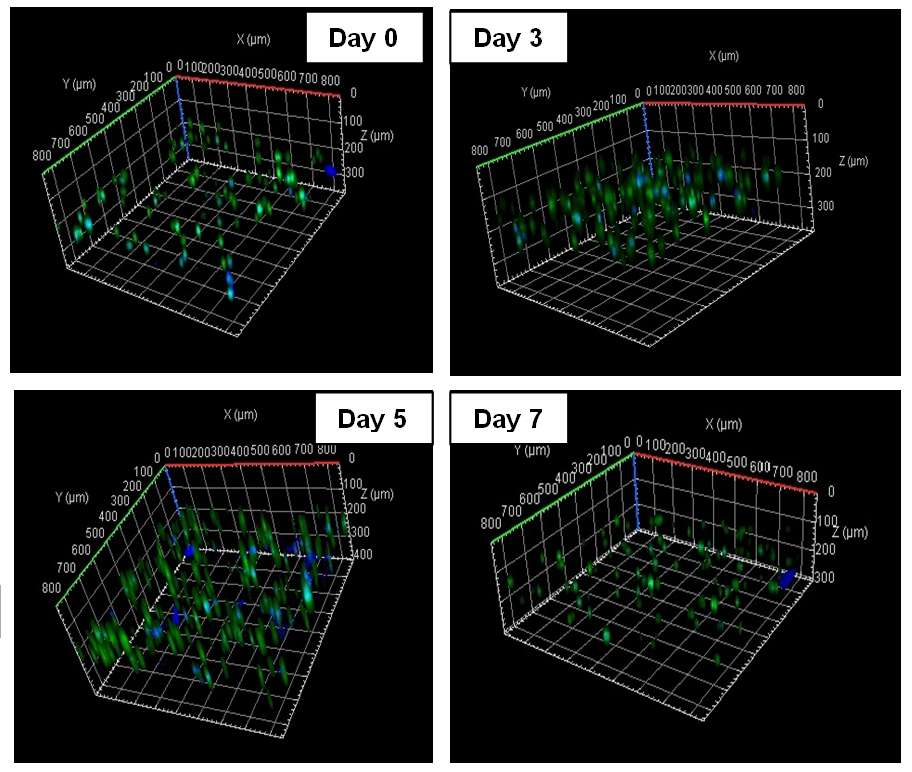
Fig.1: Confocal images of bioprinted CH based hydrogel having KC & HFF cells throughout 7 days. The number of live (green) and total number of cells (blue) were then counted with Image J software.
Maximising Foamability in Ethanol-Water Mixtures
Niall Ward-O’Brien, Anthony Ryan
Chemistry Department, University of Sheffield
Ethanol foams are a valuable medium for hand disinfection in healthcare settings, combining the useful rheological properties of gels with the pleasant tactile characteristics of liquids. But these foams often lack the robustness and smooth consistency of their aqueous counterparts. This limited foaming performance detracts from the user experiences and potentially reduces compliance.
Potential foaming formulations that might offer better performance differ in a number of respects – principally related to the type and quantity of surfactant they contain and the ratio of water to ethanol in the solvent mixture – but foam quality is also affected by other factors, such as the rate at which foam is generated. This multivariate experimental space supported the adoption of a factorial experimental design.
The foamabilities of six copolymer surfactant solutions were compared across a range of concentrations and ethanol loadings, and across a number of push speeds, using a custom-built foamability rig.
Modelling the resulting data demonstrates the importance of solvent quality in controlling surfactant behaviour – in more aqueous solutions, surfactants with a smaller hydrophobic block perform better. In ethanol-rich solutions, the inverse is true. In ethanol-poor solutions, surfactants with a large hydrophobic block are likely kinetically trapped in micelles – whereas in more ethanol-rich solutions, these bulky surfactants are more mobile and thus able to reach newly-formed interfaces in greater numbers.
The results of this experiment have provided valuable insight into the relationship between formulation characteristics and foam quality. In future, these conclusions might be used to target effective surfactants for a range of solvent conditions and maximise their efficiency.
The Oral Delivery of -Globulin using Solid Core Drug Delivery Systems (SCDDS) Prepared via Supercritical Fluid Technology (SCFT)
Adejumoke Lara Ajiboye, Vivek Trivedi
University of Greenwich, Central Avenue, Chatham Maritime, Kent, ME4 4TB
The rising interest in protein therapeutics has led to growing research in the development of novel carrier systems particularly for oral delivery. However, several barriers pose as a challenge for the use of biomolecules as oral drug therapies. SCDDS allow for immobilization of these molecules on a solid surface to improve and maintain their stability, followed by the enteric coating to achieve targeted/sustained release1. The aim of this study was to produce SCDDS using -globulin as the therapeutic drug, silica particles as core materials, fatty acids (FAs) as shell coating and SCFT as a processing technique. The adsorption of -globulin onto silica (SFP, SXDP, SAL, and MSU-H) particles was investigated against surface porosity, pH and concentration of protein. In summary, -globulin immobilization was favourable on mesoporous particles, with the maximum adsorption achieved with SFP and SXDP (511 and 387 mg/g respectively). The -globulin adsorbed- silica particles were coated with myristic acid (MA), palmitic acid (PA) and stearic acid (SA) by melt deposition in supercritical CO2 (scCO2). The use of scCO2 was established to ensure that the integrity of immobilized molecules was not compromised during the coating procedure. The in-vitro release of -globulin from SCDDS formulations was investigated in simulated gastric and intestinal conditions (SGF/ SIF), and pH 7.4 phosphate buffer. SCDDS prevented the release of -globulin in SGF, with a maximal release of 31% and 75% achieved in SIF and at pH 7.4 respectively. The stability of released -globulin evaluated by performing CD spectroscopy was found to be better for experiments carried out in SIF. These results suggest that SCDDS have the potential to protect biomolecules from the harsh gastric environment and provide release in upper intestine without causing any denaturation of the protein/peptide based pharmaceuticals.
Reference
Trivedi, V., et al., The Preparation of SCDDS for the Biomolecular Delivery, 2014.
CALL FOR ABSTRACTS
Abstract submission for consideration for a Poster presentation is 7th December 2018.
Students who submit a poster can attend for free - state that you are a student on your email from a university address when you submit your abstract. When your poster is accepted you will sent a registration code.
Abstracts must be submitted using the correct template and emailed directly to Dr. Helen Ryder at
No abstracts sent by other means (fax, post) will be accepted.
Instructions must be followed strictly and those not conforming to the required format will be disqualified.
The personal data of the presenting author must be filled in the submission form.
Abstract preparation
Abstracts should contain a sentence stating the study’s objective, a brief statement of methods, a summary of the results obtained and a statement of the conclusions. Please note that it will not be satisfactory to say ‘the results will be discussed’. Use a short and specific title. Capitalize initial letters of trade names.
Abstracts must be written in English and can not exceed 300 words.
Type your abstract using any word processor and using the template provided. Tables, graphics, figures and images can be included in the abstract. Ensure you include the title and authors in the abstract submission.
Topics
Industry 4.0 is a developing area and so we are keen to hear about a wide range of different ways in which science and technology are advanced formulation from design to consumption. Please ensure that your poster highlights the impact of Industry 4.0 on formulation and shows how your poster is either enabling Industry 4.0 or shows the impact of Industry 4.0 on business as usual.
Registration
All presenting authors must register for the meeting and pay the appropriate delegate fees.
Acceptance
Once the abstract has been submitted, the presenting author will receive an e-mail message within 3 days as confirmation of receipt stating the submission reference number. Abstracts will be reviewed following the final submission date.
Details of abstracts will appear in the program exactly as submitted. Authors are requested to follow the rules and guidelines strictly and use the template provided. Authors are solely responsible for the correctness and accuracy of the data submitted.
Abstracts will be published in the program only on receipt of registration payment in full.
Ensure that the abstract does not exceed the 300 word limit and then send to Dr. Helen Ryder by email
Cancellations
If you wish to withdraw or cancel your abstract submission then this must be done via email to Dr Helen Ryder by 5th December 2018. Abstracts not withdrawn by 5th December 2018 may appear in the program.
Files for download:
Abstract guidelines -
F4AbstractSubmissionFormGuidelines.doc
Abstract template -

