Formulation and Neutrons - a follow-up meeting to "Clever Characterisation for Smarter Formulation" - held at ISIS on 27th June 2016.
For more information on ISIS and its work with neutrons please go to the ISIS home page - http://www.isis.stfc.ac.uk/
The FSTG is very greatful to the whole ISIS team who organised a fantastic day, allowing us to almost get hands on with neutrons and certainly realise many more possiblilities for their application in smarter formulation. Please let the FSTG know if the day inspired you to use neutrons and if you can tell us what you did to help inspire others.
Introduction to ISIS – Dr Christopher Frost – Industrial Liaison Manager - link to presentation
ISIS, the UK’s neutron source (www.isis.stfc.ac.uk), is a spallation neutron source. This means that it uses high power accelerators to generate neutrons for condensed matter science rather than a fission reactors used by the other neutron source in which the UK is a partner, the Institute Laue Langevin (ILL)in Grenoble, France (see www.ill.eu ). At ISIS neutrons are freed from the nuclei of the metal tungsten by hitting it with a beam of protons travelling at 84% the speed of light.
The nature of the neutron production in spallation sources means that both the energy spectrum of neutrons and the temporal structure are different between the two; a fact exploited at both sources to optimise their respective neutron instrumentation for a whole range of science. At ISIS the pulsed nature of the production of neutrons means that the time-of-flight technique is used by the instruments; a technique that ISIS has been pioneering since it started operation some 30 years ago.
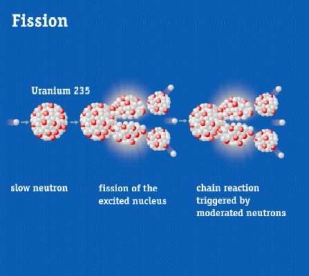
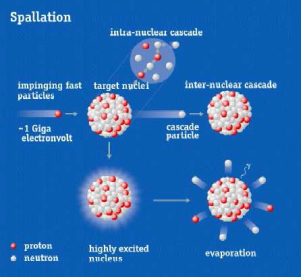
The figure shows spallation and fission neutron production
The neutrons at ISIS are ‘optimised’ by moderating their energy by collision with hydrogen containing materials so that their De Broglie wavelength is commensurate with the atomic and molecular structures found within hard and soft condensed matter. The diagram below shows the length scales accessible by neutrons in comparison with other techniques.
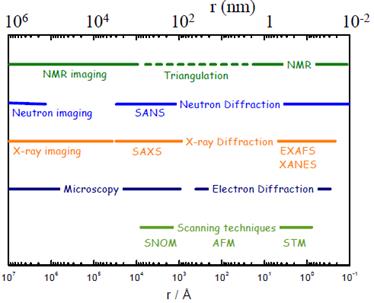
The figure shows the length scales covered by the neutron scattering techniques
Although at ISIS the neutron’s wavelike property is used for structural studies by diffraction, the mass of the neutron make it also ideal for studying the dynamics of materials through energy transfer between the system and the neutron – inelastic scattering . Below the diagram shows how the neutron can access a large region of space defined by both the size (or momentum) and the energy (or time) of the interaction. This large coverage makes the neutron a very powerful probe for many investigations of matter.
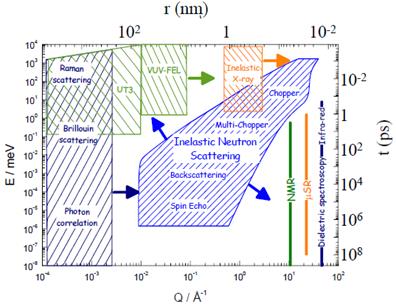
The figure shows the area covered by various neutron scattering techniques in the momentum/energy space
It is not just the wavelength and momentum scale that make the neutron extremely useful for condensed matter studies. In order for neutrons to be a useful for understanding a system there needs to be a contrast between the scattering from different elements within the structure. This then enables the neutron to be used to see differences in the structure whether by scattering, diffraction or imaging. Fortuitously, a key property of the neutron is that its interaction strength (or cross section) with individual elements (and their isotopes) does not vary in a simple way with atomic number but is random (see the diagram below). This empirical randomness contrasts with X-rays which see a steady increase in cross section with atomic mass. The neutron’s ability to effectively ‘see’ light atoms is, therefore, one of the key reasons for its use in soft condensed matter and hydrogenous systems – and why it is the perfect complementary technique to X-rays for many studies. The simple fact that hydrogen has a high neutron cross section but its isotope deuterium has a low cross section enables high contrast to be obtained in soft matter systems by deuterating one structural component i.e. the water phase, one monomer in a copolymer or one surfactant and not the other.
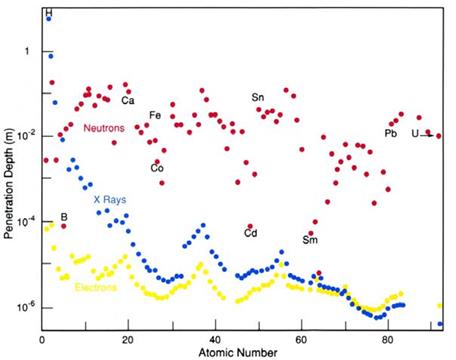
The figure shows penetration depth for neutrons (red), x-rays (blue) and electrons (yellow) through different elemental materials. For neutrons notice the that there is no obvious relationship with atomic number, for example penetration depth into boron being 100x less than carbon, iron or lead.
The interaction with neutrons is also relatively weak, so neutrons can penetrate 10s of cm into materials allowing structure within a material to be determined, and perhaps as important, sophisticated sample environment to be deployed allowing realistic environments to be achieved.
Of less relevance for soft materials, neutrons have a magnetic moment and so are able to probe subtle differences in magnetisation, key for investigations of magnetic recording media for example.
ISIS provides many routes to enable researcher both academic and industrial to exploit the world leading neutron techniques available at its facility. The academic community tends to access ISIS via a twice yearly competitive peer-reviewed route where the emphasis is on eventual publication in scientific journals.
Industry has access to this but ISIS also provides a commercial confidential proprietary route which is being actively used by several industrial sectors.
More recently, to further demonstrate the economic impact of the neutron scattering technique, ISIS instigated the Industrial Collaborative R&D route. This can be thought of as a blend of the two mentioned above. Using this route work is carried out for free at the point of access on the basis that it will be eventually published, but the subject of the work and all results are kept confidential until the results can be assessed. Once the commercial value of the research is known the commercial partners can then decide if they would like them to be kept proprietary and not to be published; if this is the case then payment is made for the experimental study. In this way ISIS hopes that the benefits of neutron studies can be explored by industry with little commercial risk, but potentially large commercial benefit.
Neutron Diffraction – Dr Sam Callear - link to presentation
The suite of neutron diffraction instruments at ISIS cover the spectrum from ordered crystalline materials to disordered liquids. The instruments optimised for the more ordered, crystallographic measurements enable the atomic structure of the sample to be determined and refined, including refinement of lattice parameters, atomic positions, atomic occupancies and isotropic/anisotropic thermal parameters. Neutron diffraction is highly complementary to X-ray diffraction, enabling different atoms to show up more clearly in the data (in particular, hydrogen atoms) as well as magnetic structure determination. Dedicated beamlines offer even greater capabilities, such as high-pressure measurements (up to 28 GPa), or stress and strain analysis. Areas of application are wide ranging from battery materials to archaeology and drugs to explosives.
At the opposite end of the spectrum, the neutron diffraction instruments at ISIS that are optimised for measuring the diffuse scattering from a sample enable the study of liquids and glasses. The data collected allows atomistic models of the structure of these disordered materials to be built, thus identifying preferred molecular conformations and interactions. These instruments can provide information from the atomistic length scale (< 1 Å) up to ~300 Å, thus enabling some larger objects, such as small micelles to be studied too. The range of scientific applications is therefore diverse from molecular liquids and solutions, colloidal dispersions and confined fluids to supercritical fluids, porous media and conducting glasses.
Measurements can be made under a variety of conditions including variable temperature (4 – 2273 K), gas handling capabilities (<1x10-3 – 200 bar) and high magnetic fields, in addition to taking time resolved measurements in order to learn about the kinetics of a process. There is also a wide range of in-situ tools available in order to collect neutron diffraction data at the same time as taking other measurements, for example thermal gravimetric analysis/dehydration & hydration/in-situ Raman spectroscopy/ionic conductivity.
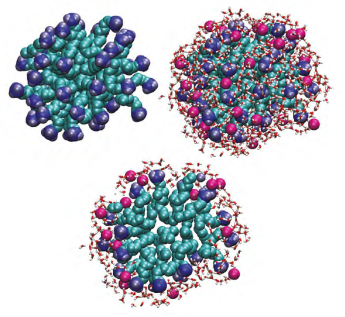
Diffraction studies of a 0.4M decyltrimethylammonium bromide (CTAB)in water identifies a micellar radius of 1.8nm and show that water molecules are only able to penetrate into the micelle as far as the bromide ion (Hargreaves et al. J. Am. Chem. Soc. 133 (2011) 16524-16536).
Imaging – Dr Christopher Frost (on behalf of Dr Winifred Kockelmann) - link to presentation
IMAT is a conventional neutron radiography and tomography instrument, but can also carry out energy-selective imaging and diffraction analysis with strain + phase being retrofitted next year.
The combination of imaging and diffraction currently being commissioned allows strain to be mapped simultaneously with imaging studies.
Energy selective imaging allows time energy radiographs to be collected which provide rich information about material structures.
Neutrons are particularly effective at imaging water ingress into complex systems either soft or hard matter either by radiography (direct imaging) or tomography. Ingress of solvent / hydrogen into materials can also be monitored.
Sample size is constrained by the design of the instrument and the need to get sufficient neutron count, so for energy selective imaging the sample size is 20cm x 20cm which gives a maximum resolution of 50um.
Neutron Spectroscopy – Dr Jeff Armstrong - link to presentation
Neutron spectroscopy allows access to the complete range of frequencies which other more commonly used spectroscopic techniques access. These range from quasi-elastic through rotations, phonons, vibration and balistic recoil – the range accessible depends on the design of the specific instrument.
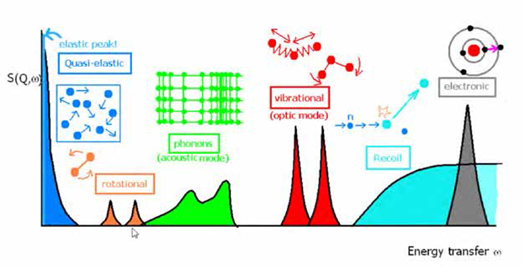
The complete range of spectroscopy that neutrons can probe, depending on the design of the instrument.
The TOSCA instrument is an example of a vibrational neutron spectrometer. Using neutrons has the great advantage of having no selection rules (i.e. get both infrared and Raman vibrations) and also having an extremely high sensitivity to hydrogen containing compounds. In addition they have the benifit of high penetrating power, thus probing the bulk of the material. In addition, with specific deutration of given molecular groups one can pin-point where a vibration is coming from.
As an example, recent examination of metal oxide frameworks and zeolites has given insights both of adsorption and topological modes which have allowed optimised structures to be designed for gas adsorption and catalysis.
OSIRS – Quasi-elastic neutron spectroscopy gives information about chain dynamics / rotations and diffusion, once again particularly powerful for hydrogen. ISIS has deep experience in using simulation to interpret the experimental results obtained. These techniques can give quantitative measures of molecular diffusion.
Large Scale Structures:
Small-Angle Neutron Scattering – Dr Sarah Rogers - link to presentation
Small-angle neutron scattering (SANS) explores lengthscales in reciprocal space by detecting the number of scattered neutrons as a function of the scattering vector, Q. Q is inversely proportional to distance.
SANS can determine the size, polydispersity, structure and interactions within a wide range of disordered materials.
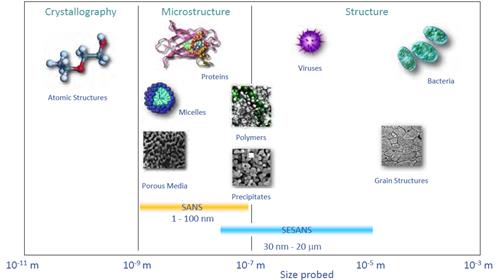
The length scales and type of materials which can be probed by SANS.
Data can be analysed in a variety of ways ranging from simple mathematical approximations such as Guinier and Porod plots to more complex mathematical modelling.
SANS is applicable for multi-component systems probed between 1 to 100nm, with SESANS extending the range to ~ 20 mm.
ISIS has a number of SANS instruments - LOQ, SANS2D, LAMOR (spin echo being added by TU Delft / NWO) and ZOOM.
Examples of SANS experiments include:
Understanding viscosity enhancement of liquid CO2 by the addition of CO2 compatible surfactants with varying counterions (Eastoe et al. Langmuir 26 (2010) 83-88).
Revealing the solution structure of the proteasome activator PA28 (Sugiyama et al. Biochem Biophys Res Commun 432 (2013) 141-145).
Studying the effect of water on engine oil additive stability (Dowding et al. Langmuir 24 (2008) 3807-3813).
Detection and understanding of transient phases formed during the crystallisation of polymers (Ungar et al. Macromolecules 38 (2005) 7201-7204).
Neutron Reflection – Dr Becky Welbourn - link to presentation
Neutron reflectrometry allows buried interfaces to be probed. These interfaces can be solid-liquid, air-liquid, liquid-liquid or air-solid. Example systems measured include biological membranes, solar cells, detergents, corrosion inhibitors and magnetic thin-films and the information retrieved can be the thickness and hydration of the layer as well as it’s roughness.
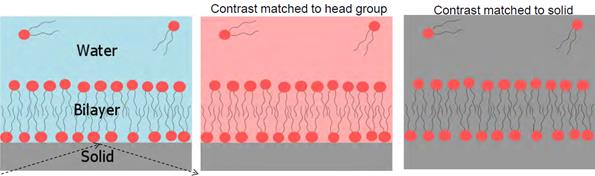
Examples of contrast matching and variation to ‘hide’ and highlight interfaces
As with other neutron scattering techniques, reflectometry experiments make great use of contrast matching and variation. This is often achieved by deuterating solvents or chemicals and is key to obtaining the information required to understand complex systems. As shown in the figure, by using contrast matching and variation, interfaces can effectively be ‘made invisible’ or can be highlighted to give a detailed picture of the system under investigation.
Examples of neutron reflectometry studies include:
Unique insights into the adsorption of surfactants at the air-water or oil-water interface (Xu et al. Langmuir 29 (2013), Zarbakhsh et al. Langmuir 21 (2005), Campana et al. Langmuir 30 (2014)).
Structural changes of polymer brushes at a solid interface with the addition of surfactant and salt as well as changes in pH (Moglianetti et al. Langmuir 27 (2011)).
Studying asymmetry in biological membranes (Clifton et al. Angew. Chem. Int. Ed. (2015)).
Kinetic studies of hydrogen loading and unloading into buried metal surface. Time slices of 1 – 10 minutes were achieved (Bannenberg et al. J Phys Chem C 120 (2016)).

