TiO2 nanoparticle dispersions studied by sedimentation analysis: Hansen Parameters vs. DLVO interpretations - poster
Lucie Delforce1, Evamaria Hofmann2, Véronique Nardello-Rataj1, Jean-Marie Aubry1
1 Univ. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, UMR 8181 – UCCS – Unité de Catalyse et Chimie du Solide, Lille, France
2 Univ. Regensburg, Department of Physical Chemistry, 93051 Regensburg, Germany
Contact Email:
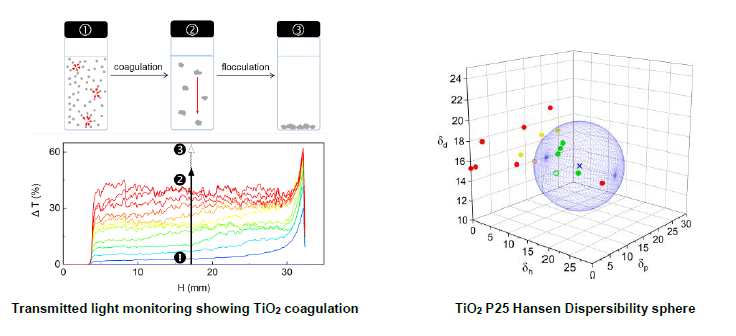
Titanium dioxide nanoparticles (TiO2 NPs) are among the most widely used NPs due to their interesting optical and catalytic properties. Most applications require their dispersion in fluid or solid matrixes. It is of first importance that NPs be and remain homogenously dispersed in the matrix to achieve optimal properties and stability. Hansen Dispersibility Parameters (HDP)1 have been shown to be an effective approach for rationalizing and predicting the stability of various types of NP dispersions including TiO21,2. However, it is not excluded that interparticle electrostatic interactions, not considered in Hansen’s approach, also play a significant role, especially for organic solvents having a notable dielectric constant3–5. In this work, we discuss the respective contributions of DLVO and non-DLVO interactions in the stability of TiO2 P25 nanoparticles dispersions, with a special emphasis on the relevance of the HDP concept to rationalize non-DLVO interactions in organic solvents.
For that purpose, zeta potential measurements in aqueous media and in 20 organic solvents are carried out to identify dispersions for which the stability can be explained by the DLVO theory from those for which the stability results from more specific NP-solvent interaction. These latter solvents are used to build the Hansen Dispersibility Sphere of TiO2 P25 with a Turbiscan as stability analyser. This light scattering method provides detailed information regarding the destabilization mechanisms of dispersions i.e. agglomeration and flocculation of NPs, which reflect the balance between interparticle attraction and electrostatic repulsion6–8.
References
(1) Süß, S.; Sobisch, T.; Peukert, W.; Lerche, D.; Segets, D. Determination of Hansen Parameters for Particles: A Standardized Routine Based on Analytical Centrifugation. Adv. Powder Technol. 2018, 29 (7), 1550–1561.
(2) Wieneke, J. U.; Kommoß, B.; Gaer, O.; Prykhodko, I.; Ulbricht, M. Systematic Investigation of Dispersions of Unmodified Inorganic Nanoparticles in Organic Solvents with Focus on the Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2012, 51 (1), 327–334.
(3) Janusz, W.; Sworska, A.; Szczypa, J. The Structure of the Electrical Double Layer at the Titanium Dioxide/Ethanol Solutions Interface. Colloids Surf. Physicochem. Eng. Asp. 1999, 152 (3), 223–233.
(4) Lyklema, J. Principles of Interactions in Non-Aqueous Electrolyte Solutions. Curr. Opin. Colloid Interface Sci. 2013, 18 (2), 116–128.
(5) Rosenholm, J. B. Evaluation of Particle Charging in Non-Aqueous Suspensions. Adv. Colloid Interface Sci. 2018, 259, 21–43.
(6) Woo, S. H.; Min Gu, L.; Rhee, C. K. Sedimentation Properties of TiO 2 Nanoparticles in Organic Solvents. Solid State Phenom. 2007, 119, 267–270.
(7) Liu, Z. Q.; Yang, X.; Zhang, Q. TURBISCAN: History, Development, Application to Colloids and Dispersions. Adv. Mater. Res. 2014, 936, 1592–1596.
(8) Luo, M.; Qi, X.; Ren, T.; Huang, Y.; Keller, A. A.; Wang, H.; Wu, B.; Jin, H.; Li, F. Heteroaggregation of CeO2 and TiO2 Engineered Nanoparticles in the Aqueous Phase: Application of Turbiscan Stability Index and Fluorescence Excitation-Emission Matrix (EEM) Spectra. Colloids Surf. Physicochem. Eng. Asp. 2017, 533, 9–19.

